Depo Provera Lawsuit: Tumor Claims
Depo Provera has been linked to a 5.5× higher risk of developing meningioma brain tumors.
Quick Facts About Depo Provera Lawsuit
If you or a loved one received Depo Provera birth control shots and developed a brain tumor, you may qualify for substantial compensation.
FREE Depo Provera CASE REVIEW
CALL NOW: 1-866-374-0338
What is Meningioma?
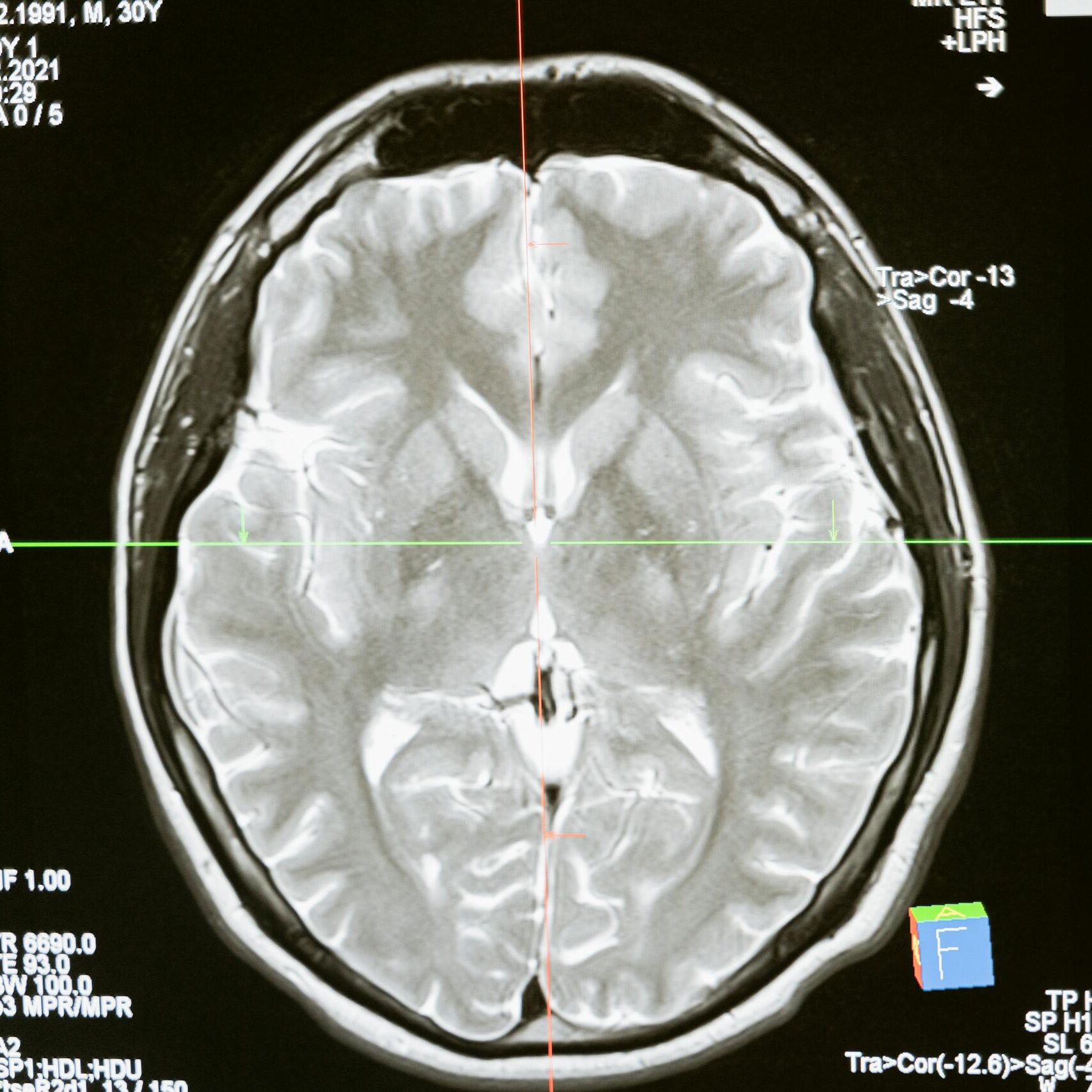
Meningioma is a type of brain tumor that develops in the membranes (meninges) surrounding the brain and spinal cord. Most meningiomas are considered benign, but they can still grow large enough to press on the brain or spinal cord, leading to serious neurological problems.
Because meningiomas often develop slowly, they may not be diagnosed until symptoms become significant and imaging such as MRI or CT scans is performed. Treatment can include surgery, radiation therapy, or, in some cases, ongoing monitoring.
The exact cause of meningiomas is not fully understood. However, recent studies have found a potential connection between long-term use of Depo-Provera (medroxyprogesterone acetate) and a higher risk of developing these tumors. Lawsuits are now investigating this association and seeking accountability for patients affected.
Do You Qualify for a Depo Provera Brain Tumor Lawsuit?
Medical Criteria
Most claimants meet at least one of the following:
Preferred Documentation
If you don’t yet have these, our team can help you secure them
Have you or your loved one been diagnosed with Meningioma? Get a free case review →
Have You Experienced These Symptoms?
Persistent or worsening headaches
Seizures or convulsions
Blurred, double, or partial vision loss
Memory lapses or cognitive decline
Hearing loss or ringing in the ears
Balance problems or limb weakness
ALERT: Untreated or undiagnosed meningiomas can grow and cause permanent neurological damage. Act now to protect your rights.
Depo Provera Lawsuit MDL Timeline
From FDA approval through MDL consolidation, here are the key milestones in the Depo Provera Lawsuit (meningioma) litigation.
1992 – FDA approves Depo Provera
FDA approval
2024 – Landmark BMJ study reports 5.55× higher meningioma risk
BMJ study (Roland N et al., 2024)
Feb 2025 – MDL 3140 centralized in northern Florida
JPML centralization order (MDL No. 3140)
December 2025 – FDA Label Update
Read more
Harmed by Depo Provera?
Get answers. Get help. Start your free brain-tumor case review.
or
With over 30 years of experience and more than $100 million recovered, The Johnson Law Firm has stood up to major drug manufacturers and won. We’re proud to represent clients across the U.S. in high-stakes litigation involving defective medications.
Real Results From Real Clients
“I was amazed with the results that JLF achieved for me. I wasn’t expecting anything like that, but it was just awesome. They just went to bat for me and accomplished quite a bit. I was just really blown away by the results.”
— Julie
How a Depo Provera Brain Tumor Lawsuit Works
Depo Provera (medroxyprogesterone acetate) was approved in 1992, but a landmark 2024 BMJ study and other medical research have shown long-term users face a significantly higher risk of developing meningioma brain tumors. When drug makers fail to update warnings or downplay emerging science, they can be held liable under various product-liability theories.
Results-Driven Representation: Our team will marshal the latest science, review your medical records at no cost, and fight for the maximum compensation you deserve.
How We Help Depo Provera Brain Tumor Victims
At The Johnson Law Firm, we take a strategic, compassionate approach to every Depo Provera brain-tumor case. Our goal is to uncover the full truth, hold negligent drug makers accountable, and secure maximum compensation for the harm you’ve endured.
Our Process:
Results-Driven Representation: Focus on your health while we fight for maximum recovery.
