1/10/2007 – FDA Notice Recall Expanded
This letter is intended to inform you of actions being undertaken by Davol, a subsidiary of C. R. Bard, Inc.,
The Bard Composix Kugel Mesh Hernia Patch Recall is a patch used in the repair of hernias. The patch has caused dangerous complications in some patients.
The Bard Composix Kugel Mesh Hernia Repair Patch Recall has been upgraded by the United States Food and Drug Administration to “Class I” because the defect associated with the use or exposure to the Bard Composix Kugel Mesh Large Patch has a reasonable probability to cause serious adverse health consequences, including death.
The FDA issued an immediate recall of Bard Composix® Kugel Mesh Hernia Repair Patches on December 22, 2005 and updated the list again on January 10, 2007. (See the Recall Notices and Affected Device List Below).
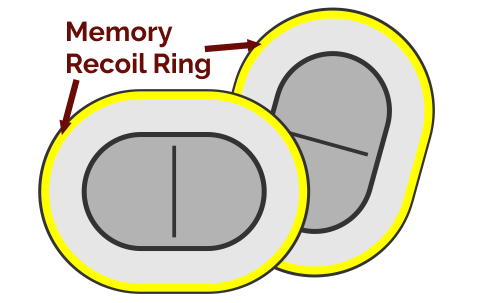
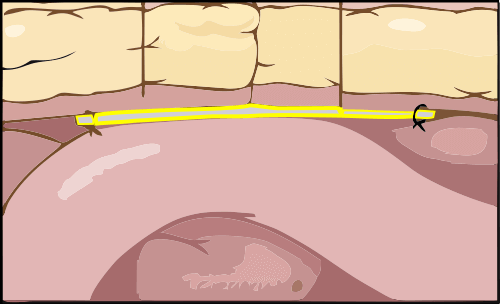
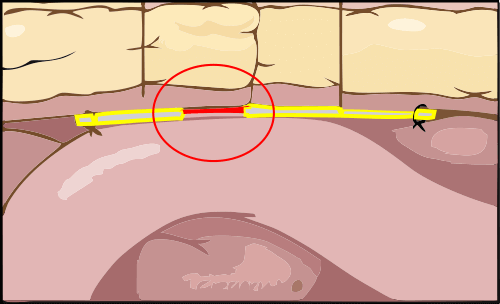
The “memory recoil ring” that opens the Composix Kugel Mesh Patch after it has been inserted into the intra-abdominal space can break. This can lead to bowel perforations and/or chronic intestinal fistulae (abnormal connections or passageways between the intestines and other organs).
The FDA has advised patients who have received this device to seek medical attention immediately if abdominal pain, fever, tenderness at the implant site or other unusual symptoms occur. The FDA has instructed surgeons and hospitals to immediately stop using the recalled products and return the unused patches to the company.
We have expanded our investigation to include non-recalled hernia mesh, hernia patches, and hernia mesh patches manufactured by Davol, Inc. and Bard. We are now evaluating claims for patients with
| Product Code | Description | Size | Date Recalled |
| 10206 | Bard ® Composix ® Kugel | Extra Large Oval, 8.7” x 10.7” | December 2005 and January 2006 |
| 10207 | Bard ® Composix ® Kugel | Extra Large Oval, 10.8” x 13.7” | December 2005 and January 2006 |
| 10208 | Bard ® Composix ® Kugel | Extra Large Oval, 7.7” x 9.7” | December 2005 and January 2006 |
| 10209 | Bard ® Composix ® Kugel | Oval, 6.3” x 12.3” | 24-Mar-06 |
| 10202 | Bard ® Composix ® Kugel | Large Oval, 5.4” x 7” | March 24, 2006 and updated Jan. 11, 2007 |
| 10204 | Bard ® Composix ® Kugel | Large Circle, 4.5” | March 24, 2006 and updated Jan. 11, 2007 |
| 10203 | Bard ® Composix ® Kugel | Small Circle, 7.6 cm | Not-recalled to date however defects have been reported and claims are being evaluated. |
| 10201 | Bard ® Composix ® Kugel | Small Oval, 8 cm x 12 cm | Not-recalled to date however defects have been reported and claims are being evaluated. |
| 10205 | Bard ® Composix ® Kugel | Medium Oval, 11 cm x 14 cm | Not-recalled to date however defects have been reported and claims are being evaluated. |
| Bard® Kugel® Hernia Patch | All sizes | Not-recalled to date however defects have been reported and claims are being evaluated. | |
| Bard® VentraleX® Hernia Patch | All sizes | Not-recalled to date however defects have been reported and claims are being evaluated. | |
| Bard CK Parastomal Patch | All sizes | Not-recalled to date however defects have been reported and claims are being evaluated. | |
| Bard® Modified Kugel™ Patch | All sizes | Not-recalled to date however defects have been reported and claims are being evaluated. | |
| Bard® Composix® E/X Mesh | All sizes | Not-recalled to date however defects have been reported and claims are being evaluated. |
We have represented clients in every state and helped thousands of injured clients nationwide make recoveries for their injuries. We are ready to help you! Our clients are never at risk of paying any fees, expenses, or costs out of pocket. All fees costs and expenses are paid only out of any recovery we make for you.
Do not delay. Contact us now to find out if we can help. The claim evaluation is free. All claims have strict time limitations which will bar any recovery you or your loved may be entitled if the claim is not brought on time.
2/1/2007 – FDA – Recall classified as Class I because the defect associated with the use or exposure to the Bard Composix Kugel Mesh Large Patch has a reasonable probability to cause serious adverse health consequences, including death. Customers should stop using the recalled product and return unused units to the company.
The Composix® Kugel Mesh Patch is used to repair ventral (incisional) hernias caused by thinning or stretching of scar tissue that forms after surgery. The patch is placed behind the hernia defect through a small incision. The patch is then held open by a “memory recoil ring” that allows the patch to be folded for insertion and later spring open and lay flat once it is in place.
Davol, Inc., Sub. C.R. Bard, Inc.
100 Sockanossett Crossroad
Cranston, RI 02920
The “memory recoil ring” that opens the Composix® Kugel Mesh Patch can break under the stress of placement of the large sized products in the intra-abdominal space. This can lead to bowel perforations and/or chronic intestinal fistulae (abnormal connections or passageways between the intestines and other organs).
This letter is intended to inform you of actions being undertaken by Davol, a subsidiary of C. R. Bard, Inc.,
The firm has received numerous inquiries from patients who have non-recalled Kugel mesh patches and have had complications related to
MURRAY HILL, NJ — C. R. Bard, Inc. (NYSE-BCR) today announced that it is voluntarily recalling its Bard Composix Kugel
We provide free Case Evaluations. Please complete the following form to describe your case.
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.